Background and Significance
In sickle cell disease (SCD), a single point mutation in the beta-globin gene results in hemoglobin S (HbS) production, which can polymerize upon deoxygenation. Polymerization distorts the red blood cell (RBC) membrane and generates sickled RBCs, contributing to microvascular occlusions, which may present as acute, painful vaso-occlusive episodes (VOEs). For patients (pts) with SCD, 70% of emergency department visits and 90% of hospitalizations are VOE-related. 1,2 Despite existing treatments for VOE prevention, considerable morbidity and mortality remain; acute VOE treatment is limited to supportive care due to a lack of targeted therapies.
Accumulating nonclinical data suggest a multimodal role for complement dysregulation in SCD pathophysiology, including in vaso-occlusion, hemolysis, inflammation, thrombogenicity, endothelial activation, and end-organ damage. 3 Crovalimab is a novel, engineered, anti-complement C5 monoclonal antibody that allows for small-volume subcutaneous (SC) injection after an initial intravenous (IV) loading dose. 4 Here, we describe two randomized, double-blind, placebo-controlled trials evaluating crovalimab in pts with SCD: CROSSWALK-a (Phase 1b; NCT04912869) and CROSSWALK-c (Phase 2a; NCT05075824).
Study Design and Methods
CROSSWALK-a evaluates the safety of crovalimab in managing acute uncomplicated VOEs. CROSSWALK-c evaluates the efficacy and safety of crovalimab as adjunct therapy in preventing VOEs.
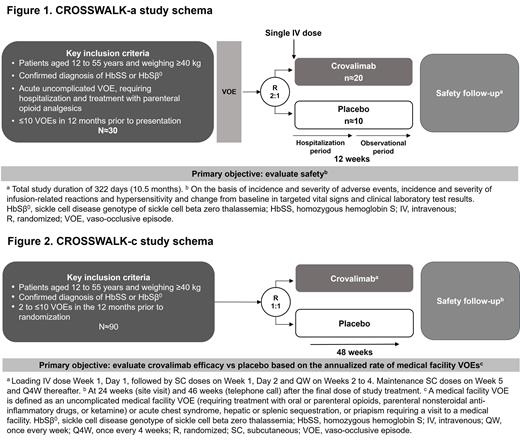
Pts 12-55 years old, weighing ≥40 kg with a confirmed diagnosis of sickle cell anemia (homozygous HbSS or heterozygous HbS/β 0 thalassemia) and vaccinated against N. meningitidis, H. influenzae type B and S. pneumoniae are eligible (Fig. 1 and 2). Concurrent SCD-directed therapies are allowed.
CROSSWALK-a
Pts must present with an acute uncomplicated VOE requiring hospitalization and parenteral opioid analgesics, and have had ≤10 VOEs in the 12 months prior to randomization. Eligible pts will be randomized 2:1 to receive a single IV weight-based tiered dose of crovalimab or placebo, and monitored during hospitalization and in an 84-day observational period followed by a safety follow-up period for a total study duration of 322 days (10.5 months).
The primary objective of CROSSWALK-a is to evaluate safety up to Day 84, including:
Incidence and severity of adverse events
Incidence and severity of infusion-related reactions and hypersensitivity
Change from baseline in targeted vital signs and clinical laboratory test results
Efficacy, pharmacokinetic (PK), pharmacodynamic (PD), immunogenicity, and exploratory biomarker endpoints will also be evaluated.
CROSSWALK-c
Pts with 2-10 VOEs in the 12 months prior to randomization are eligible. Eligible pts will be randomized 1:1 to receive weight-based tiered doses of crovalimab or placebo, consisting of initial loading doses (IV: Day 1; SC: Day 2 and weekly for Weeks 2-4) and monthly maintenance SC doses Weeks 5-49 for 48 weeks of treatment.
The primary objective of CROSSWALK-c is to evaluate crovalimab efficacy vs placebo up to 48 weeks, on the basis of:
The annualized rate of medical facility VOEs
Key secondary efficacy objectives are:
Annualized rate of home VOEs
Annualized rate of acute chest syndrome
Annualized rate of days hospitalized for medical facility VOEs
Pt-reported fatigue in adults
Safety, PK, PD, immunogenicity, and exploratory biomarker endpoints will also be evaluated.
Primary results will be published upon trial readout.
References
1. Ballas et al, Am J Hematol 2005; 79:17-25.
2. Lanzkron et al , Am J Hematol 2010; 85:797-799.
3. Roumenina et al, Am J Hematol 2020; 95:456-464.
4. Röth et al, Blood 2020; 135:912-920.
Disclosures
Salvino:F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau; OrphanDrugs: Consultancy; Abbvie: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; Gilead: Honoraria, Speakers Bureau; ABHH: Membership on an entity's Board of Directors or advisory committees; SBTMO: Membership on an entity's Board of Directors or advisory committees. Chite Asirwa:MSD: Consultancy, Honoraria; F. Hoffman-La Roche: Honoraria, Research Funding; GBT: Research Funding; Pfizer: Research Funding; Genentech Inc.: Honoraria, Research Funding; Bausch: Research Funding; Celgene: Research Funding; Millennium: Honoraria; AstraZeneca: Honoraria; Merck: Honoraria; Novartis: Honoraria; ASCO: Membership on an entity's Board of Directors or advisory committees; ESMO: Membership on an entity's Board of Directors or advisory committees; ECSACO: Membership on an entity's Board of Directors or advisory committees. Chonat:Amgen: Consultancy, Research Funding; Roche/Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda Pharmaceuticals: Consultancy, Research Funding; GBT/Pfizer: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Alexion: Consultancy, Other, Research Funding. De Franceschi:Agios: Research Funding; Bristol Myers Squibb: Research Funding; F. Hoffmann-La Roche Ltd, Basel: Membership on an entity's Board of Directors or advisory committees. Eleftheriou:Pfizer: Honoraria; GBT: Honoraria; Alexion: Honoraria; Forma Therapeutics: Honoraria; Agios: Honoraria. Mahlangu:Roche: Research Funding; Pfizer: Research Funding; Spark Therapeutics: Research Funding; Catalyst: Research Funding; Sanofi: Research Funding; Sandoz: Research Funding; Novo Nordisk: Research Funding; BioMarin Pharmaceutical Inc.: Research Funding. Nur:VERTEX: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau; Novartis: Consultancy, Research Funding, Speakers Bureau. Charania:F. Hoffmann-La Roche Ltd, Basel: Current Employment. Gradinaru:F. Hoffmann-La Roche Ltd, Basel: Current Employment, Current equity holder in publicly-traded company. Hsia:Genentech Inc.: Current Employment. Luder:F. Hoffmann-La Roche Ltd, Basel: Current Employment. Perretti:F. Hoffmann-La Roche Ltd, Basel: Current Employment. Srekovic:F. Hoffmann-La Roche Ltd, Basel: Current equity holder in publicly-traded company, Ended employment in the past 24 months; Genentech Inc.: Current Employment, Current equity holder in publicly-traded company. Bartolucci:Addmedica: Consultancy, Membership on an entity's Board of Directors or advisory committees; INNOVHEM: Current equity holder in private company; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bluebird: Consultancy; Roche: Consultancy; Emmaus: Consultancy; GBT: Consultancy; Jazz Pharma: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal